You've probably been there. You are staring at a beaker, a pile of white powder, and a flask of water, wondering if "a pinch" actually matters. In the world of chemistry, it matters a lot.
Basically, the concentration of solute in solution is just a way of saying how much "stuff" is crammed into a specific amount of "liquid." But the math gets messy fast. If you're mixing saline for a medical IV or just trying to get your saltwater aquarium's salinity right, "close enough" can be a disaster.
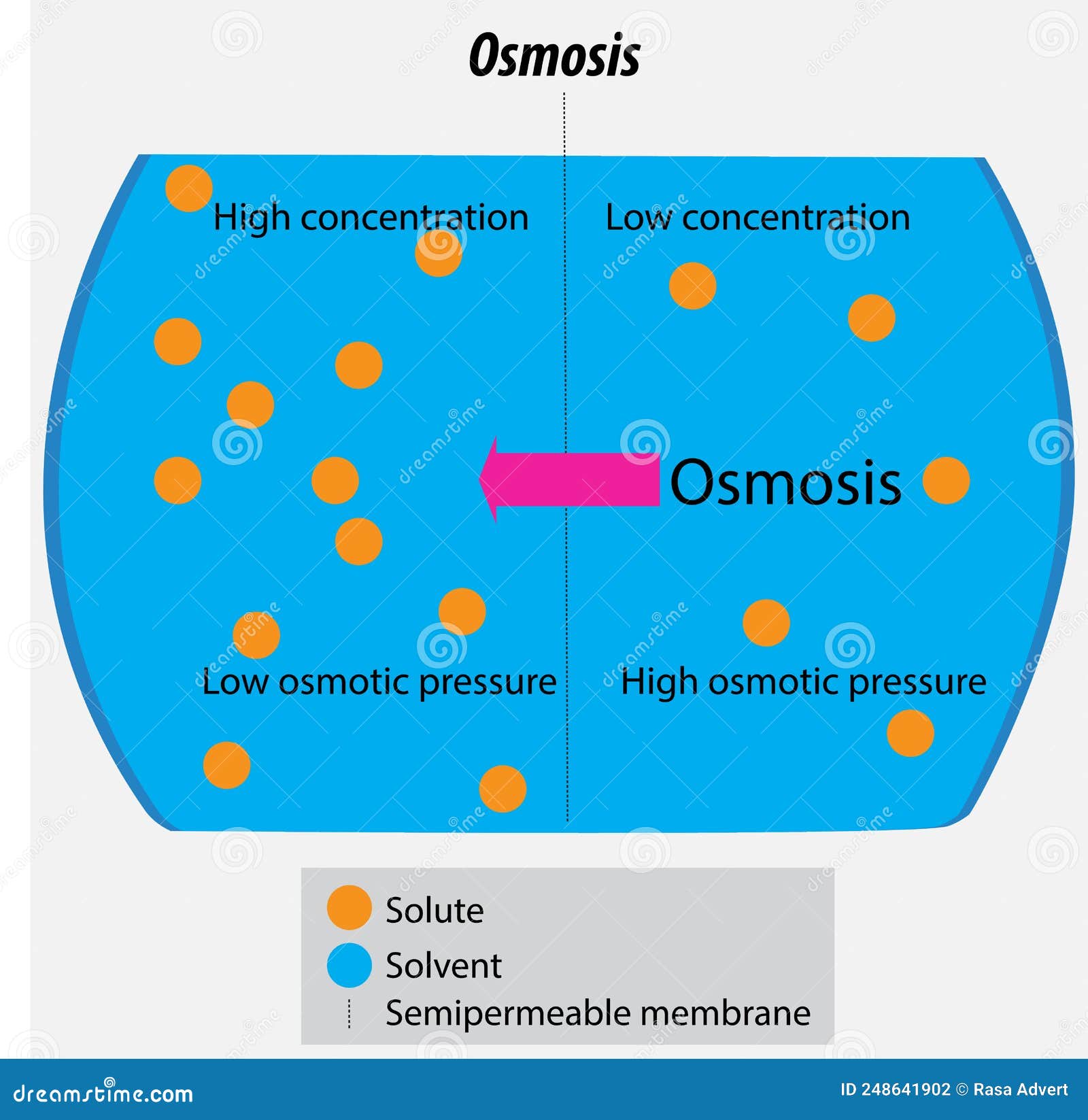
Solute is the thing being dissolved (like salt). Solvent is the thing doing the dissolving (usually water). Together, they make the solution. It sounds simple until you realize there are about six different ways to measure it, and using the wrong one is how lab accidents happen.
Why Molarity Isn't Always the Answer
Most students gravitate toward Molarity ($M$) because it's the poster child of chemistry textbooks. It’s defined as moles of solute per liter of solution.
👉 See also: The Best Ways to Repeat a YouTube Video Without Losing Your Mind
But here’s the kicker: Molarity is temperature-dependent. If you measure a 1.0 M solution in a freezing lab in January and then use that same solution in a sweltering warehouse in July, the concentration has technically changed. Why? Because liquids expand when they get hot. The volume increases, but the number of moles stays the same. Suddenly, your "precise" measurement is a lie.
If you need real precision regardless of the weather, you look at Molality ($m$). Note the "l." Molality is moles of solute per kilogram of solvent. Since mass doesn't change when the temperature rises, Molality stays rock solid. It's why researchers like Marie Curie or modern analytical chemists at places like NIST (National Institute of Standards and Technology) rely on mass-based measurements when things get serious.
The Mass Percent Confusion
Sometimes, you don't need moles. If you're working in a kitchen or a soap-making shop, you're likely looking at mass percentage. This is simply (mass of solute / total mass of solution) × 100.
People mess this up constantly. They divide by the mass of the water, not the total mass. If you add 10g of salt to 100g of water, your total mass is 110g. Your concentration isn't 10%. It's actually about 9.09%. That 1% difference might not ruin your soup, but it could definitely ruin a chemical reaction meant to stabilize a volatile compound.
The Weird World of Parts Per Million (ppm)
When we talk about lead in drinking water or CO2 in the atmosphere, the concentration of solute in solution is so tiny that percentages are useless. 0.00015% is a pain to write.
So, we use ppm. Think of it like this: one ppm is essentially four drops of ink in a 55-gallon drum of water. When the EPA sets limits on arsenic in your tap water (which is 0.010 ppm, by the way), they are dealing with trace amounts that can still have a massive biological impact.
Dilution: The Art of Watering It Down
You have a stock solution that is way too strong. You need it weaker. The formula $M_1V_1 = M_2V_2$ is your best friend here.
🔗 Read more: External Hard Drive for iPad: Why Most People Are Still Doing It Wrong
But there’s a safety rule every chemist has burned into their brain: Add Acid to Water (AA). If you are trying to change the concentration of a concentrated acid by adding water directly into it, the reaction can be so exothermic (releasing heat) that it literally boils the water instantly, spraying acid all over your face. By adding the solute (the acid) to the solvent (the water) slowly, the water can absorb the heat. It’s a physical reality of how molecules interact at the surface level.
Why Saturation is a Hard Wall
You can't just keep adding sugar to tea forever. Eventually, the tea says "enough" and the sugar just sits at the bottom. This is the saturation point.
The concentration of solute in solution at this point is at its maximum for that specific temperature. If you heat the tea up, you can dissolve more. This creates a "supersaturated" solution. If you've ever made rock candy, you’ve used this trick. You dissolve a ton of sugar in boiling water, then let it cool. The water "holds" more sugar than it should, and the second you drop a string in there, the sugar crashes out of the solution and crystallizes.
Real-World Impact: The Dead Sea
A perfect natural example of extreme concentration is the Dead Sea. It has a salinity of about 34%. Compare that to the ocean’s average of 3.5%. The concentration is so high that the water is denser than the human body, which is why you can’t help but float. But it's also toxic to almost all life. There’s a fine line between a solution that supports life and one that is literally too thick to allow biology to function.
How to Calculate Concentration Without Losing Your Mind
If you're trying to figure this out at home or in a lab, follow these steps.
💡 You might also like: Amazon Kindle Covers Paperwhite: Why Most People Choose the Wrong One
- Identify your units. Are you working with grams, milliliters, or moles? If the bottle says "mol," you're in Molarity territory. If it says "%," it’s likely mass/volume or mass/mass.
- Account for the total. Always remember that the "solution" is the solute plus the solvent. If you forget to add them together for your denominator, your math is wrong before you even start.
- Check the temperature. If you’re doing high-precision work, make sure your liquids are at room temp (usually 20°C or 25°C).
- Convert early. Don't try to mix milligrams and liters. Turn everything into grams and liters (or whatever your target unit is) before you touch the calculator.
Actionable Next Steps
To master the concentration of solute in solution, stop thinking about it as an abstract math problem and start viewing it as a ratio of physical objects.
If you're in a lab setting, always double-check whether your procedure calls for $w/v$ (weight/volume) or $v/v$ (volume/volume). For DIY projects like mixing nutrients for hydroponics, buy a cheap TDS (Total Dissolved Solids) meter. It measures electrical conductivity to give you a rough ppm reading instantly.
Finally, if you are mixing anything hazardous, always calculate your volumes before you open the containers. Prevention is better than a chemical spill.
Keep your ratios tight and your measurements cold. The difference between a breakthrough and a mess is usually just a decimal point in your concentration calculation.