You’re standing in a bathroom after a hot shower. The mirror is a foggy mess. You wipe a streak through the droplets, and it’s cold to the touch, but beneath that surface level observation, there’s a massive energy transfer happening that most people completely flip-flop in their heads. When people ask is condensing endothermic or exothermic, they usually get tripped up because they associate "hot" with "exothermic" and "cold" with "endothermic" in a way that doesn't quite fit the physics.
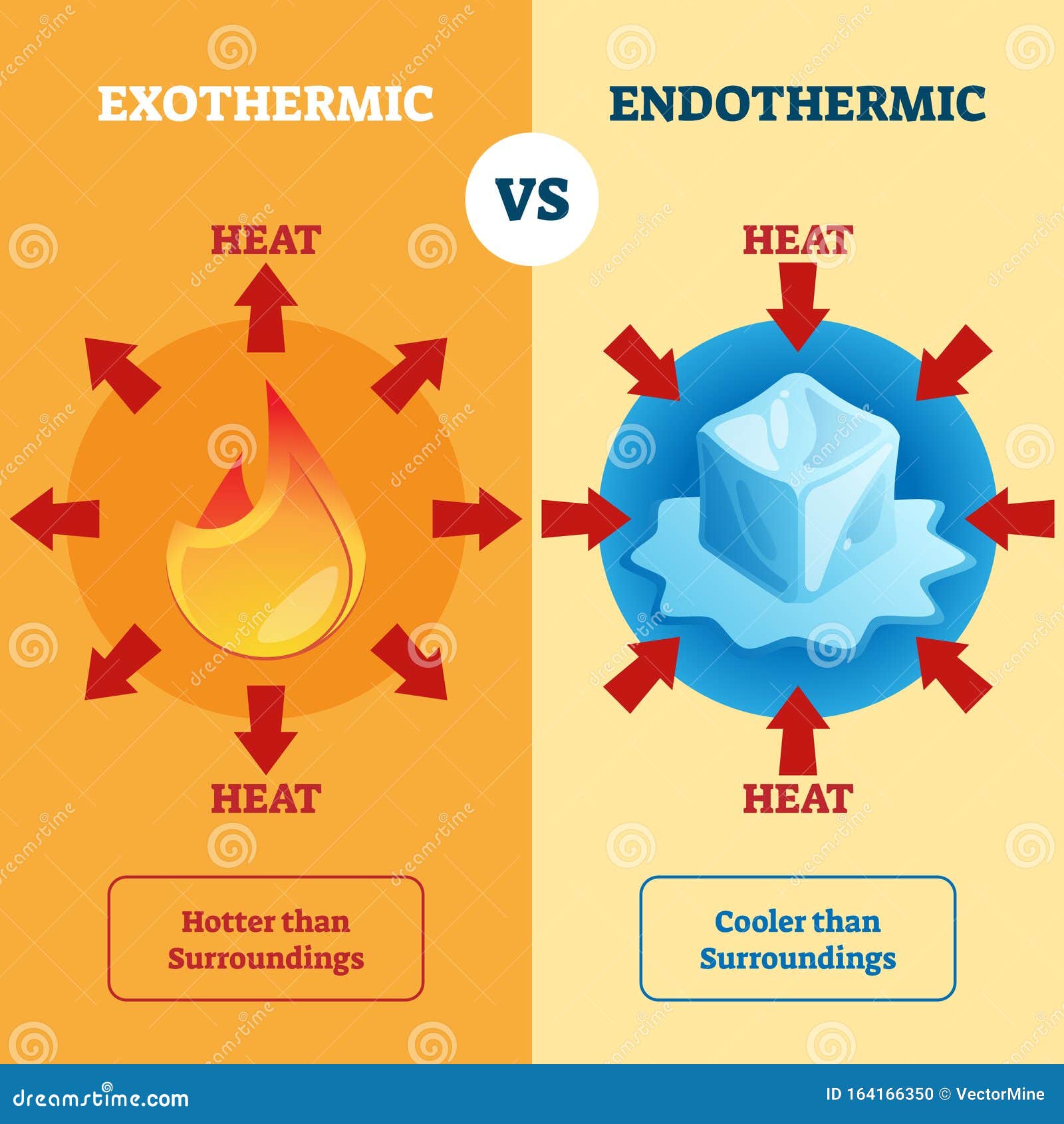
Here is the bottom line: Condensation is exothermic.
It’s not just a little bit exothermic; it’s a powerhouse of energy release. When gas turns into a liquid, it has to ditch a ton of kinetic energy to slow down those zippy molecules enough to make them stick together. That energy doesn't just vanish into a void. It gets dumped into the surroundings.
📖 Related: De donde es este numero de telefono: Cómo rastrear llamadas extrañas sin perder la cabeza
Why the Energy Shift Happens
Think about water vapor. Those molecules are high-energy rebels. They are flying around, bouncing off walls, and staying as far apart as possible. To get them to settle down into a liquid state—condensation—you have to remove the energy that’s keeping them apart.
When that gas hits a cooler surface, like your window on a winter morning or the outside of a soda can, the molecules lose their heat. This heat is transferred out of the substance. In chemistry and physics, any process that releases heat into the environment is exothermic.
Honestly, it's kinda wild when you think about the scale. To turn one gram of water vapor at 100°C into one gram of liquid water at the same temperature, the system releases about 2,260 Joules of energy. This is known as the latent heat of vaporization (or condensation, depending on which way you're headed). That’s a massive amount of energy being shoved out into the air just to change the state of the matter without even changing the temperature of the substance itself.
The Bond-Making Secret
Most people remember from high school chemistry that breaking bonds takes energy. That's endothermic. But we often forget the flip side: making bonds releases energy.
Even though we aren't talking about "chemical" bonds in the sense of creating a new molecule like $CO_2$, we are talking about intermolecular forces. In water, these are primarily hydrogen bonds. As the vapor cools, these attractive forces take over. As these "bonds" or attractions form between the water molecules to create a liquid drop, they release energy.
It’s exothermic because the final state (liquid) has less internal energy than the starting state (gas). The "leftover" energy has to go somewhere. It goes to you. It goes to the mirror. It goes to the atmosphere.
Real-World Consequences of the Exothermic Nature of Condensation
This isn't just a trivia question for a chemistry quiz. The fact that condensation is exothermic literally dictates how our planet survives and how our technology functions.
Take hurricanes, for example.
A hurricane is basically a giant heat engine fueled by the exothermic nature of condensation. Warm ocean water evaporates, rising into the atmosphere as vapor. As it rises, it cools and condenses into clouds. Because condensation is exothermic, it releases a staggering amount of heat into the upper atmosphere. This heat warms the surrounding air, making it more buoyant, causing it to rise faster, which sucks up more moisture, creating a feedback loop of pure power. Without this specific exothermic release, a hurricane would just fizzle out.
Steam Burns Are Worse Than Water Burns
You've probably heard that a burn from steam is way more dangerous than a burn from boiling water, even if they are both at 100°C. Why? Because of the exothermic transition.
When boiling water hits your skin, it transfers heat as it cools down. But when steam hits your skin, it first has to condense into liquid water. Because condensation is exothermic, it dumps that massive "latent heat" directly into your tissue before the water even begins to cool down as a liquid. You're getting hit with a double dose of thermal energy. It’s a brutal demonstration of physics in action.
Common Misconceptions: Why Do We Get Confused?
The confusion usually stems from how we feel. When we sweat, we feel cool. That’s because evaporation is endothermic—it sucks heat away from our skin to turn the sweat into gas.
💡 You might also like: The Real Definition for Social Networking and Why Most People Still Get It Wrong
Naturally, we assume the opposite must "feel" hot. But we rarely "feel" condensation happening in a way that registers as heat because it’s usually happening on a cold surface. We see the cold surface and think "Endothermic!" but we are looking at the cause, not the result. The water vapor is the thing losing the energy; the surface it lands on is the thing gaining it.
If you were the size of a molecule sitting on that mirror, you’d feel a sudden blast of warmth as the vapor turned to liquid around you.
Breaking Down the Vocabulary
- Exothermic: Energy exits the system. (Condensation, Freezing, Deposition).
- Endothermic: Energy enters the system. (Evaporation, Melting, Sublimation).
If you’re trying to remember this for a test or just to sound smart at a dinner party, just think: Gas to Liquid = Giving off Heat. ## Industrial Applications
Engineers spend their entire careers obsessing over this. In a steam power plant or a nuclear reactor, the "condenser" is a critical component. They have to use massive amounts of cooling water from rivers or cooling towers just to soak up the exothermic energy released when the "spent" steam is turned back into liquid water so it can be pumped back to the boiler.
If they didn't manage that exothermic release, the whole system would overheat and fail.
Similarly, in your refrigerator, the "coils" on the back or bottom are where the refrigerant condenses. If you touch those coils while the fridge is running, they feel hot. That’s the exothermic heat of condensation being dumped out of the inside of your fridge and into your kitchen. You’re literally feeling the "exit" of heat.
The Final Verdict on Is Condensing Endothermic or Exothermic
So, next time you see dew on the grass or fog on your glasses, remember that you're witnessing a tiny heat-release event. The environment is getting a microscopic bit warmer because those water molecules decided to stop flying and start sticking.
Condensation is exothermic. It releases energy, forms intermolecular bonds, and powers everything from the cooling of your home to the most violent storms on Earth.
What to do with this info:
- Check your AC unit: If the exterior fan is blowing cold air while the AC is on, something is wrong. It should be blowing warm air because it's releasing the heat captured during condensation and the cooling cycle.
- Kitchen Safety: Respect the steam. Now that you know it carries extra "latent heat" because of its exothermic transition, use tongs and stay clear of that lid when you're checking the pasta.
- Weather Watching: When you see massive thunderheads forming (cumulonimbus clouds), you're looking at a visual representation of millions of gallons of water undergoing an exothermic reaction. That’s the "fuel" for the lightning and wind to follow.
The world is just a series of energy handshakes. Condensation is just water's way of saying "I don't need this extra energy anymore—you take it."
Next Steps for Deep Learning:
- Research the Clausius-Clapeyron equation to see how pressure changes the temperature at which this exothermic transition occurs.
- Look into Heat Pipes in modern laptop cooling—they use the cycle of evaporation (endothermic) and condensation (exothermic) to move heat away from your CPU at incredible speeds.