Ever looked at your smartphone and wondered why it doesn't weigh five pounds? Or why your electric car can actually make it to the next state without dying? It all comes back to a single, lonely electron. Seriously. When we talk about the li number of valence electrons, we're basically talking about the "on-off switch" for modern portable energy.
Lithium is the third element on the periodic table. It’s light. It’s soft enough to cut with a butter knife. But its chemistry is defined entirely by the fact that it has exactly one valence electron.
That single electron is a wanderer. It wants to leave. Because that electron is so loosely held, Lithium is incredibly reactive. It’s the reason why you can't just toss a lithium battery into a shredder without sparking a localized inferno. Understanding how that one electron moves is the difference between a dead battery and a Tesla that hits 60 mph in two seconds.
The Simple Math of Lithium's Shells
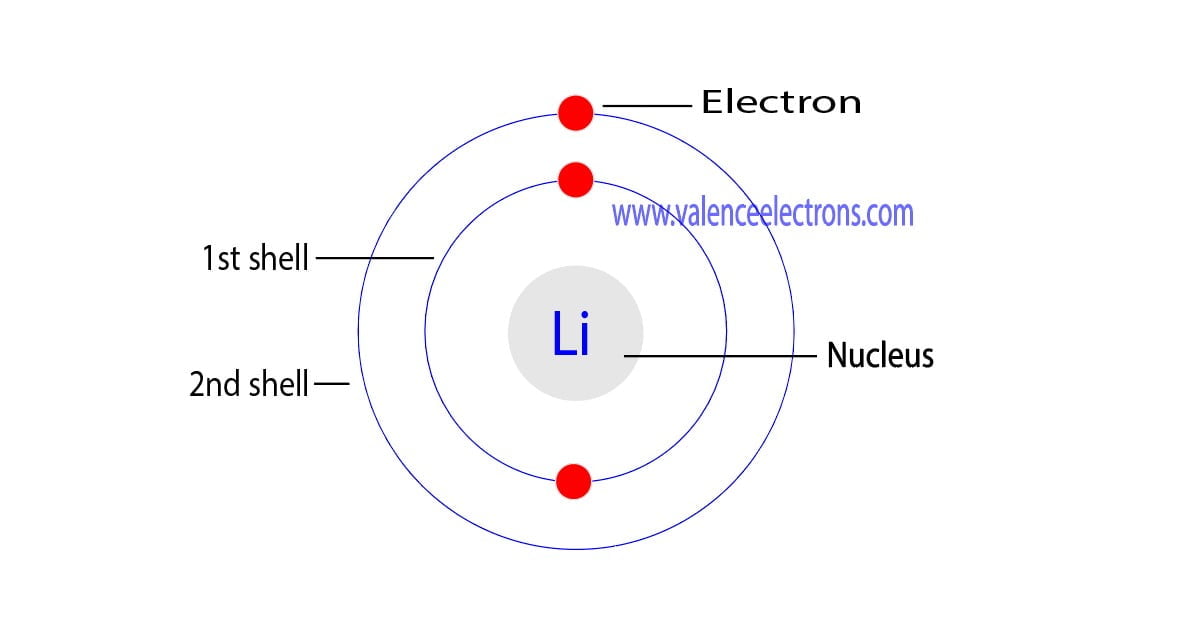
Atomic structure isn't as scary as high school chemistry made it seem. Lithium has an atomic number of 3. This means it has three protons and, in its neutral state, three electrons. But those electrons aren't just swarming around in a chaotic cloud. They follow a very specific seating chart called shells.
✨ Don't miss: Radial Distance From the Central Axis: Why This Measurement Rules the Physical World
The first shell, the one closest to the nucleus, is full with just two electrons. These are "core" electrons. They're happy. They're stable. They aren't going anywhere. This leaves the third electron all by itself in the second shell (the 2s orbital).
That lonely occupant is the li number of valence electrons.
Because atoms generally want a full outer shell to feel "stable" (the Octet Rule), Lithium has two choices: find seven more electrons to fill the second shell, or just get rid of that one outlier. Honestly, losing one is way easier than finding seven. This makes Lithium a "Group 1" alkali metal. It spends its whole existence looking for a way to ditch that electron and become a $Li^+$ ion.
Why the Li Number of Valence Electrons Matters for Your iPhone
If Lithium held onto its electrons tightly, your phone wouldn't work. The entire principle of a Lithium-ion battery relies on the "revolving door" of that single valence electron.
When you charge your phone, you're using electricity to force those lithium ions into one side of the battery (the anode). They sit there, packed in, waiting for the chance to let go of their valence electron and move back to the other side (the cathode). When you unplug and start scrolling TikTok, you're opening the gate. The lithium atoms release that one valence electron, which flows through your phone's circuits as electricity, while the lithium ion itself travels through the electrolyte inside the battery.
The Energy Density Factor
Lithium is the lightest metal. Since it only needs to move one electron to create a current, and the atom itself is tiny, you get a massive amount of "bang for your buck" in terms of weight.
- Lead-Acid Batteries: Heavy, clunky, used in gas cars.
- Nickel-Cadmium: Better, but "memory" issues and toxic.
- Lithium: The gold standard because of that single, easy-to-toss valence electron.
The Periodic Table Context: Group 1 Neighbors
Lithium isn't alone in its "one electron" lifestyle. It sits at the top of the Alkali Metals column. Hydrogen is above it (though it's a bit of a weirdo), and Sodium (Na) is right below it.
You’ve probably seen those videos of people throwing chunks of Sodium into lakes. It explodes. Why? Because Sodium has a valence electron that is even further from the nucleus than Lithium's. It's even more desperate to leave. Lithium is reactive, sure, but it's the "tame" one of the group. This relative stability—combined with its low atomic mass—is exactly why we use it for technology instead of, say, Francium, which would probably melt your house if it touched a drop of water.
The electron configuration for Lithium is $1s^2 2s^1$. That "2s1" is the smoking gun. It tells scientists exactly how this element will behave in a fire, in a battery, or even in a psychiatric medication.
Lithium in Medicine: A Different Kind of Charge
It’s kind of wild that the same element in your power drill is also used to treat bipolar disorder. While the exact mechanism is still being debated by neuroscientists like those at the National Institute of Mental Health (NIMH), the leading theory involves—you guessed it—that valence electron and the resulting ion.
Because the lithium ion ($Li^+$) is a small, positively charged particle, it can mimic other ions in your brain, like Sodium or Potassium. It essentially "mucks up" the signaling pathways that lead to manic episodes. It’s a literal physical stabilizer at the atomic level. If the li number of valence electrons were two or three, the size and charge of the ion would be completely different, and it wouldn't be able to "pass" as a sodium ion in your neurons.
Common Misconceptions About Lithium's Structure
People often get confused between the total number of electrons and the valence number. I see it all the time on student forums.
- "Lithium has 3 valence electrons." No. It has 3 total electrons. Only 1 is in the outer shell.
- "Lithium is a gas." Nope. It’s a solid metal, though it’s so light it actually floats on oil.
- "The valence electron is always lost." Usually, yes. But Lithium can share it in covalent bonds, though it’s much more "ionic-leaning" in its personality.
When Lithium bonds with something like Fluorine to make Lithium Fluoride, it’s a total hand-off. Lithium says, "Take this electron, please," and Fluorine (which has 7 valence electrons and desperately needs 1) says, "Gladly." They both end up with full shells and a very strong electrostatic attraction to each other.
How to Calculate Valence Electrons for Any Element
If you can find the element on the periodic table, you can find the valence number. For the main group elements (the "tall" columns on the left and right), it’s just the column number.
- Group 1 (Lithium, Sodium, etc.): 1 valence electron
- Group 2 (Beryllium, Magnesium): 2 valence electrons
- Group 13 (Boron, Aluminum): 3 valence electrons
- Group 17 (The Halogens): 7 valence electrons
It gets a little messy in the middle with the transition metals (the "short" columns), but for Lithium, it's as straightforward as it gets.
The Future: Beyond the Single Electron?
Engineers are currently obsessing over "Lithium-Sulfur" or "Solid-State" batteries. The goal is always the same: how do we get more power out of the li number of valence electrons without the battery catching fire?
The problem with Lithium's reactivity is that if the battery gets too hot, that "desire" to lose the electron can lead to a "thermal runaway." The atom is so eager to react that it starts a chain reaction. Current research is focusing on better electrolytes—the "highway" the ions travel on—to make sure that single valence electron only moves when we want it to.
Real-World Implications of Atomic Weight
Because Lithium is the lightest solid element, it has a high "specific capacity." In plain English, that means it can store a lot of energy per kilogram. If we used Lead (which also has valence electrons to give), your Tesla battery would weigh as much as a blue whale. We are stuck with Lithium because of the fundamental physics of its second electron shell. There is no "lighter" way to move electrons around in a solid state.
Summary of Lithium's Atomic Profile
To keep it simple, here is the breakdown of what makes Lithium tick:
- Atomic Number: 3
- Total Electrons: 3
- Valence Electrons: 1
- Shell Configuration: 2, 1
- Reactivity: High (it really wants to be $Li^+$)
- Primary Use: Energy storage and mood stabilization
Honestly, it's pretty poetic. The simplest metal on earth is the one we rely on for our most complex technology. Without that 2s1 electron hanging out on the edge of the atom, we'd still be tethered to wall outlets and carrying around heavy, inefficient lead-acid bricks.
Actionable Steps for Students and Tech Enthusiasts
If you're trying to master the concept of valence electrons or just want to understand your tech better, here is what you should do next:
- Visualize the Shell: Draw a circle with a "3P" in the middle. Draw a small circle around it with 2 dots, then a larger circle with 1 dot. That's your Lithium atom.
- Check Your Battery Health: Now that you know lithium-ion batteries work by moving that one electron back and forth, you know that "cycles" matter. Every time those ions move, the physical structure of the battery degrades slightly. Keep your phone between 20% and 80% charge to reduce the "stress" on those lithium ions.
- Periodic Table Navigation: Find Lithium (Li) on a periodic table. Look at the column. Then look at the column for Oxygen (Group 16). Since Oxygen has 6 valence electrons and needs 2, and Lithium has 1 to give, you can immediately see why the formula for Lithium Oxide is $Li_2O$. It takes two Lithium atoms to satisfy one Oxygen.
- Safety First: Remember that Lithium's reactivity is a direct result of its electron count. Never puncture a lithium battery. When the internal barrier breaks, those valence electrons find a path they aren't supposed to take, releasing all that stored energy as heat instantly.
The li number of valence electrons isn't just a trivia fact for a chemistry quiz. It's the literal foundation of the digital age. By mastering this one tiny detail, you understand the core of chemical bonding, electricity, and even pharmacology.