Ever think about why you don't just fall through your chair? Or why your phone screen actually glows when you tap it? It’s basically all thanks to one incredibly tiny, almost invisible number. Honestly, the mass of electron is one of those fundamental constants that sounds boring in a high school textbook but is secretly the MVP of the universe.
It's small. Like, incomprehensibly small.
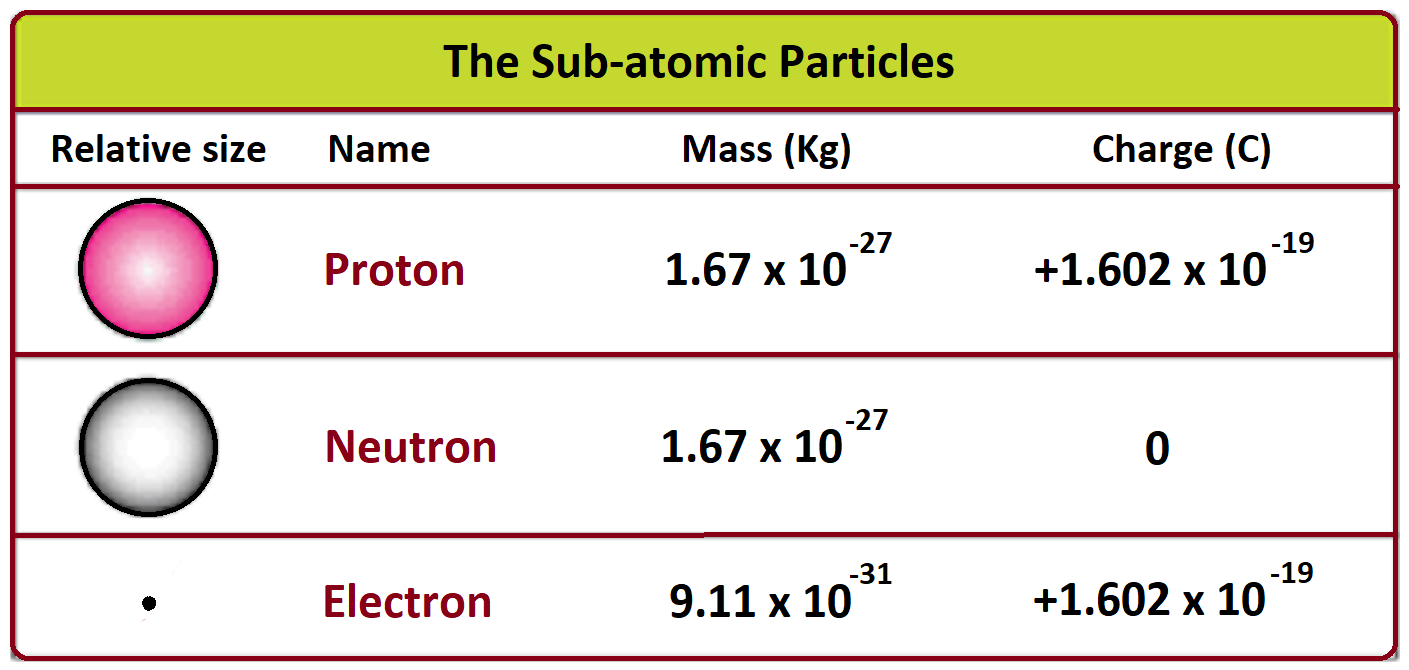
If you tried to weigh an electron on a kitchen scale, you'd be waiting forever. Even if you had the world's most sensitive lab equipment, you aren't "weighing" it in the traditional sense. We’re talking about a value that sits at approximately $9.1093837 \times 10^{-31}$ kilograms. That’s a decimal point followed by 30 zeros before you even hit a digit. It’s a ghost of a particle. Yet, if that number shifted by even a tiny fraction, the stars would burn out, atoms wouldn't hold together, and you definitely wouldn't be sitting here reading this.
The Number That Defined Modern Physics
When J.J. Thomson discovered the electron in 1897 using his cathode ray tube experiments, he didn't actually know the mass. He knew the ratio. He could see how much these "corpuscles," as he called them, bent in magnetic and electric fields. He realized they were way lighter than a hydrogen atom—about 1,800 times lighter, actually. But the specific mass of electron remained a bit of a mystery until Robert Millikan came along with his famous oil-drop experiment.
📖 Related: Is Discord Down? How to Use Down Detector for Discord Without Losing Your Mind
Millikan was clever. He sprayed tiny drops of oil into a chamber and used an electric field to stop them from falling. By balancing the force of gravity with the electric force, he figured out the charge of a single electron. Once you have the charge and the charge-to-mass ratio, the math just clicks. You get that $9.11 \times 10^{-31}$ kg figure we all use today.
It’s worth noting that when we talk about this mass, we are usually referring to the "rest mass." In the world of Einstein’s relativity, things get weird when they move fast. Since electrons in particles accelerators move at a significant fraction of the speed of light, they actually "gain" mass relative to an observer. But for almost every calculation in chemistry or electronics, the rest mass is the golden standard.
Why the Ratio to Protons Matters
Chemistry is essentially a long-running drama about electrons moving from one place to another. The fact that the mass of electron is so much smaller than the mass of a proton (about 1/1836th, to be precise) is why atoms have the structure they do.
Think about it this way.
The protons and neutrons are the heavy, stubborn anchors in the center. They stay put. The electrons are the light, zippy messengers that do all the work. Because they are so light, they can be shared, traded, or pushed through a copper wire to power your toaster. If electrons were as heavy as protons, they wouldn't zip around in clouds; they’d be sluggish. Bonding would be impossible. Life? Forget about it.
Quantum Mechanics and the "Size" Myth
One thing that trips people up is trying to imagine how big an electron is based on its mass. In our daily lives, if something has mass, it has a size. A bowling ball is bigger than a marble. But the electron is a "point particle." As far as we can tell with our current technology—including the Large Hadron Collider—the electron has no detectable spatial extent. It’s a point.
🔗 Read more: Apple All In One Desktop Computer: What People Get Wrong About the iMac
This creates a bit of a brain-melter. You have a finite mass of electron packed into a zero-volume point. In classical physics, that would mean infinite density, which makes the math scream. This is where quantum mechanics steps in to save the day (and our sanity). We treat the electron not as a hard little ball, but as a wave-particle duality. Its mass is distributed in a probability cloud.
Real-World Consequences of the Mass of Electron
You might think this is all just theoretical stuff for people in white lab coats. Wrong. The specific value of the mass of electron dictates the "Bohr radius," which is the average distance between the nucleus and the electron.
This distance determines the size of every atom.
- Electronics: The "effective mass" of an electron changes when it moves through a crystal lattice of a semiconductor. Engineers have to account for this to build faster microchips.
- Superconductivity: At very low temperatures, electrons pair up (Cooper pairs). Their mass and charge allow them to glide without resistance.
- Medical Imaging: Your local hospital's MRI machine and PET scanners rely on the behavior of electrons and their counterparts (positrons).
If the mass of electron were slightly different, the energy levels of atoms would shift. Light would be emitted at different frequencies. The "fine structure constant," which governs the strength of electromagnetic interactions, would change. Basically, the universe's "software" would crash.
Measuring the Immeasurable
How do we know we’re right? Science isn't just taking Millikan's word for it from a hundred years ago. We’ve gotten better. Much better.
Modern physicists use Penning traps. These are devices that hold a single electron using a combination of magnetic and electric fields. By measuring the "cyclotron frequency"—how fast the electron circles in the trap—scientists can determine the mass of electron with staggering precision. The latest CODATA recommended value is refined down to a level of uncertainty that would make a Swiss watchmaker weep.
Common Misconceptions About Subatomic Weight
People often ask: "If an electron has mass, does it have gravity?"
Yes. Technically. But it’s so weak it’s laughable. The electromagnetic force between two electrons is about $10^{42}$ times stronger than the gravitational pull between them. Gravity only matters when you pile up trillions upon trillions of atoms (like a planet). At the subatomic level, mass is more about inertia—how hard it is to move the particle—than it is about pulling things toward it.
📖 Related: Hard Drive Components: What’s Actually Inside That Metal Box
Another weird one: "Does the electron lose mass when it gives off energy?"
Sorta. In an atom, an electron in a high-energy state doesn't weigh more in a way that’s easy to measure, but the system (the atom) has a mass-energy equivalence. When an electron drops an energy level and spits out a photon, the total mass of the atom decreases by a microscopic amount ($E=mc^2$). But the fundamental rest mass of electron itself stays constant. It’s a universal building block.
How to Visualize the Scale
It's hard to wrap your head around $10^{-31}$ kg. Let's try a different perspective.
If an electron weighed as much as a single penny, a proton would weigh as much as a large bowling ball. If you wanted to make a single gram of "electron stuff," you would need more electrons than there are stars in the observable universe. It is the ultimate lesson in "small but mighty."
Actionable Takeaways for Science Enthusiasts
If you're looking to apply this knowledge or dive deeper, here is how you can actually use this info:
- Calculate Atomic Scale: Use the mass of the electron to understand why certain materials are better conductors. Look up "effective mass in semiconductors" to see how engineers manipulate this value to make your computer faster.
- Explore Quantum Chemistry: If you're a student, don't just memorize $9.11 \times 10^{-31}$. Try plugging it into the Rydberg formula. Seeing how the mass directly affects the color of light emitted by a hydrogen atom makes the physics feel real.
- Stay Updated on Metrology: The SI units (like the kilogram) were redefined in 2019 to be based on physical constants rather than physical objects. Follow the National Institute of Standards and Technology (NIST) to see how they continue to refine these measurements.
- Think Critically About Material Science: Next time you see a "miracle material" like graphene, remember that its properties come from how electrons—with their specific mass—behave in that 2D space.
The mass of electron isn't just a number in a database. It’s the reason your heart beats, your lights turn on, and the ground stays solid under your feet. It’s the tiny anchor of reality.