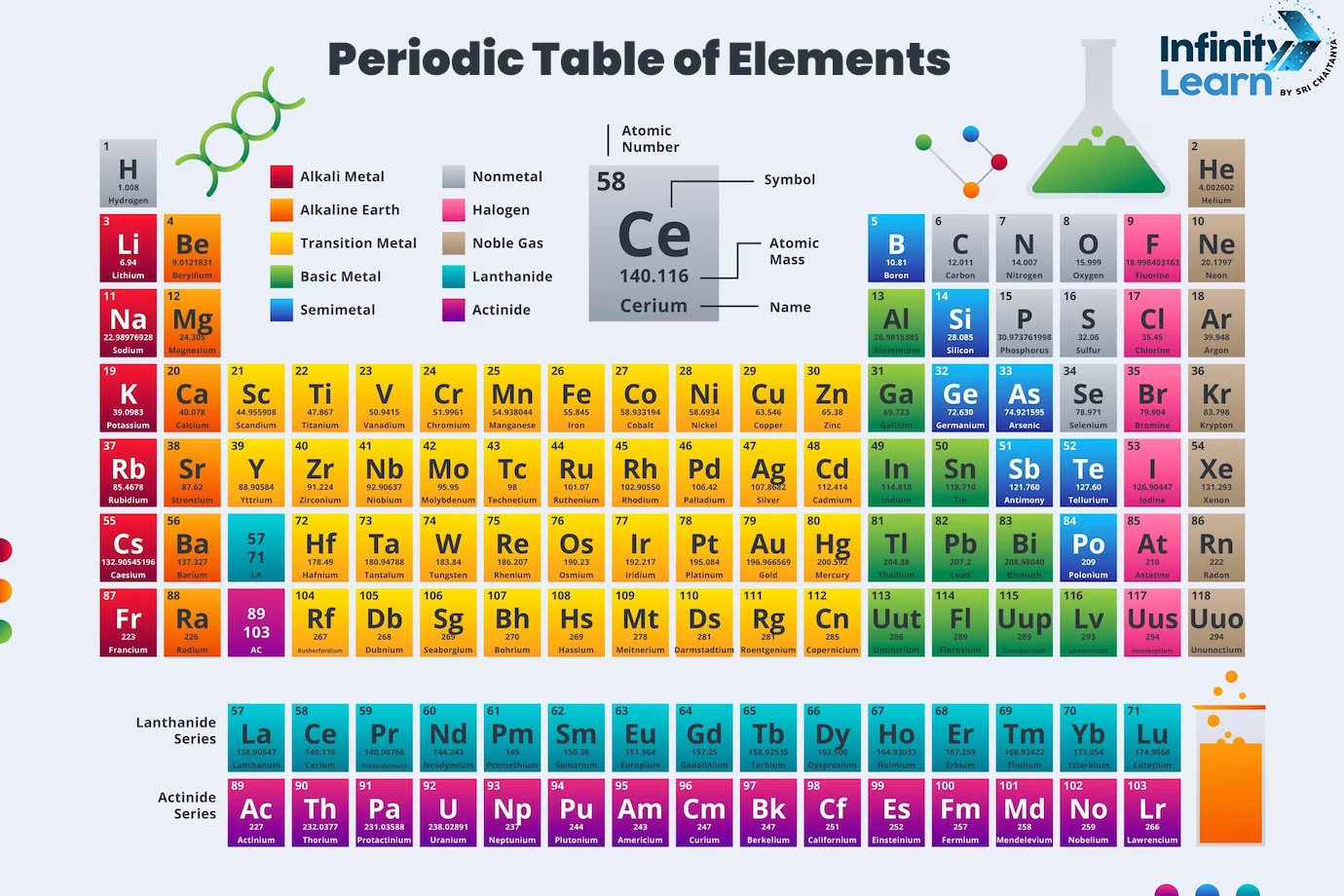
You’d think naming something as fundamental as a building block of the universe would be a straightforward, dignified process. It isn't. Honestly, the history of naming chemical elements is a chaotic mix of ancient mythology, bitter Cold War rivalries, and nerdy inside jokes. If you’ve ever wondered why lead is "Pb" or why there’s a sudden cluster of elements named after places in California and Russia at the bottom of the periodic table, you're looking at the scars of scientific history.
It’s messy.
The Wild West of Discovery
Back in the day, if you found an element, you basically owned the naming rights. There were no committees. No IUPAC (International Union of Pure and Applied Chemistry) to tell you "no." If you were a chemist in the 18th century, you just picked a name that sounded cool or described the smell. Take Bromine. It comes from the Greek bromos, which literally just means "stink." Antoine Jérôme Balard discovered it in 1826 and basically decided the world needed to know his new liquid element smelled like a swamp.
Then you have the ancient stuff. Gold, silver, iron—they’ve been around since humans first started hitting rocks with other rocks. Their names are linguistic fossils. The symbol for Iron is Fe because of the Latin ferrum, but the word "iron" itself has Germanic roots. We’re essentially using a multi-layered linguistic cake every time we look at a periodic table.
When Countries Fight Over Atoms
The real drama started when we hit the synthetic era. This is where naming chemical elements turned into a geopolitical boxing match. During the Cold War, labs in the United States (Lawrence Berkeley National Laboratory) and the Soviet Union (Joint Institute for Nuclear Research) were racing to synthesize heavy elements.
They both claimed to have found Element 104 first.
The Americans wanted to call it Rutherfordium.
The Soviets wanted Kurchatovium.
This wasn't just about science; it was about national pride and legacy. This "Transfermium Wars" era lasted for decades. It got so petty that IUPAC eventually had to step in and create a formal set of rules just to keep everyone from shouting at each other in peer-reviewed journals. They even tried using temporary, neutral names like "Unnilquadium," which sounds like something out of a bad sci-fi novel. Eventually, a compromise was reached in 1997, and Rutherfordium stuck for 104, while the Soviets got Dubnium (Element 105) to honor their research site in Dubna.
The Modern Rulebook (And How to Break It)
Today, you can't just name an element after your cat. IUPAC has strict categories. You have to pick from five specific buckets:
- A mythological concept or character (including astronomical objects).
- A mineral or similar substance.
- A place or geographical region.
- A property of the element.
- A scientist.
Even with these rules, people get creative. Take Tennessine (117). It’s named after the state of Tennessee because of the contributions of Oak Ridge National Laboratory. Then there’s Oganesson (118), named after Yuri Oganessian. He’s one of only two people to have an element named after them while they were still alive. Imagine the flex of having your own spot on the periodic table while you're still eating breakfast. The other was Glenn Seaborg (Seaborgium), who famously said the honor meant more to him than a Nobel Prize.
The Problem with "Ium"
Everything ends in "-ium." It’s the law. Or it was, until recently. Halogens have to end in "-ine" (like Tennessine) and noble gases have to end in "-on" (like Oganesson). This helps keep the table organized, but it also makes the newer elements sound a bit more uniform and, frankly, a bit less poetic than "Mercury" or "Phosphorus."
Phosphorus is a great story, by the way. Hennig Brand discovered it in 1669 while trying to turn human urine into gold. He didn't find gold, but he found something that glowed in the dark. He named it after the Greek word for "light-bearer." It’s a beautiful name for a discovery that started in a bucket of pee.
💡 You might also like: How to extract images from pdf without losing quality (The easy way)
Why the Symbols Don't Always Make Sense
If you're a student or just a curious person, the symbols are the most frustrating part of naming chemical elements. Why is Tungsten W? Because the Germans call it Wolfram. Why is Antimony Sb? Because of the Latin stibium.
We’re stuck with these because of "priority." In science, the first person to publish generally gets the credit, and the name that sticks in the literature is hard to scrub out. It’s a legacy system. It’s like why your keyboard is QWERTY—it’s not the most efficient way to do things, but everyone already learned it, so we’re stuck with it for eternity.
The Ytterby Obsession
If you want to win a trivia night, you need to know about Ytterby. It’s a small village in Sweden. Just a regular place with a quarry. But that one quarry yielded minerals that led to the discovery of four different elements:
- Yttrium
- Terbium
- Erbium
- Ytterbium
The chemists were clearly running out of ideas. They just kept slicing the name of the village into smaller and smaller pieces. It’s the ultimate example of how local geography dominates the periodic table.
🔗 Read more: Why the Narrows Bridge Collapse 1940 Still Haunts Engineering Today
Mythological Roots and Star Power
A huge chunk of the table is just a tribute to ancient gods.
- Promethium is named after Prometheus, who stole fire from the gods. Fitting for a radioactive element.
- Thorium is named after Thor.
- Tantalum comes from Tantalus, a Greek figure punished by standing in water he could never drink. Tantalum is chemically inert and "dislikes" reacting with other things, so the name is a bit of a nerdy pun.
Then you have the space connection. Helium was actually found on the Sun (via spectroscopy) before it was found on Earth. Hence, Helios. Tellurium (Earth) and Selenium (Moon) form a planetary pair. Scientists have always looked at the stars when trying to label the fundamental bits of the dirt beneath their feet.
Misconceptions About the Naming Process
A common myth is that the Discoverer has total control. They don't. They propose a name, but IUPAC puts it out for a public comment period. Other scientists can object if the name is too similar to something else or if it’s offensive in another language.
There's also the "Technetium" problem. For a long time, there was a gap in the periodic table at number 43. People claimed to have found it and called it "Masurium," but they couldn't prove it. When it was finally synthesized in 1937, it became Technetium (from the Greek for "artificial"). It was the first element to be man-made. The naming here was a statement: we aren't just finding nature anymore; we’re building it.
The Future of Naming
We are currently at the end of the seventh row of the periodic table. If (or when) we find Element 119, it will start the eighth row. The race is on between labs in Japan, Russia, and the US.
What will they call it?
Maybe something honoring a female scientist? The table is historically male-dominated. Only Meitnerium (Lise Meitner) and Curium (Marie and Pierre Curie) explicitly honor women, though you could argue for others via mythology. There’s a strong push in the scientific community to use future naming opportunities to fix this historical imbalance.
Actionable Insights for Science Enthusiasts
If you're looking to get deeper into the world of elemental history or perhaps you're a student trying to memorize this chaos, here is how to actually approach it:
- Learn the Latin roots first. If you understand argentum (silver), aurum (gold), and plumbum (lead), the "weird" symbols on the table suddenly make sense. It’s not a random code; it’s just old Latin.
- Follow the IUPAC Provisional Recommendations. If you’re a real chemistry nerd, you can actually read the public proposals for new element names on the IUPAC website. They post these months before they become official.
- Check out the "Periodic Table of Videos" by the University of Nottingham. Sir Martyn Poliakoff provides incredible context on the naming and discovery of each element that goes far beyond a textbook.
- Trace the geography. Map out the elements named after places (Americium, Francium, Germanium, Californium). It tells a story of where the scientific power was concentrated during the 19th and 20th centuries.
- Don't just memorize. Understand the "why." Why is Cobalt named after "kobolds" (goblins)? Because miners thought the ore was cursed since it released toxic fumes when smelted. Those stories stick better than a flashcard.
Naming chemical elements isn't a finished project. It's a living history. Every time we smash atoms together and manage to keep them in existence for a fraction of a second, we get the chance to add a new word to the human dictionary. It’s a weird, political, funny, and deeply human endeavor. Next time you look at a periodic table, don't see it as a static chart. See it as a graveyard of old arguments and a map of human curiosity.