Chemistry isn't always about complex proteins or massive polymers. Sometimes, the most influential stuff is incredibly tiny. Take the formula for carbon tetrachloride. It's just five atoms. One carbon, four chlorines. That’s it. In scientific notation, we write it as $CCl_4$.
It sounds boring, right? But this little molecule has a history that reads like a thriller—full of lifesaving breakthroughs, toxic scandals, and a global environmental rescue mission.
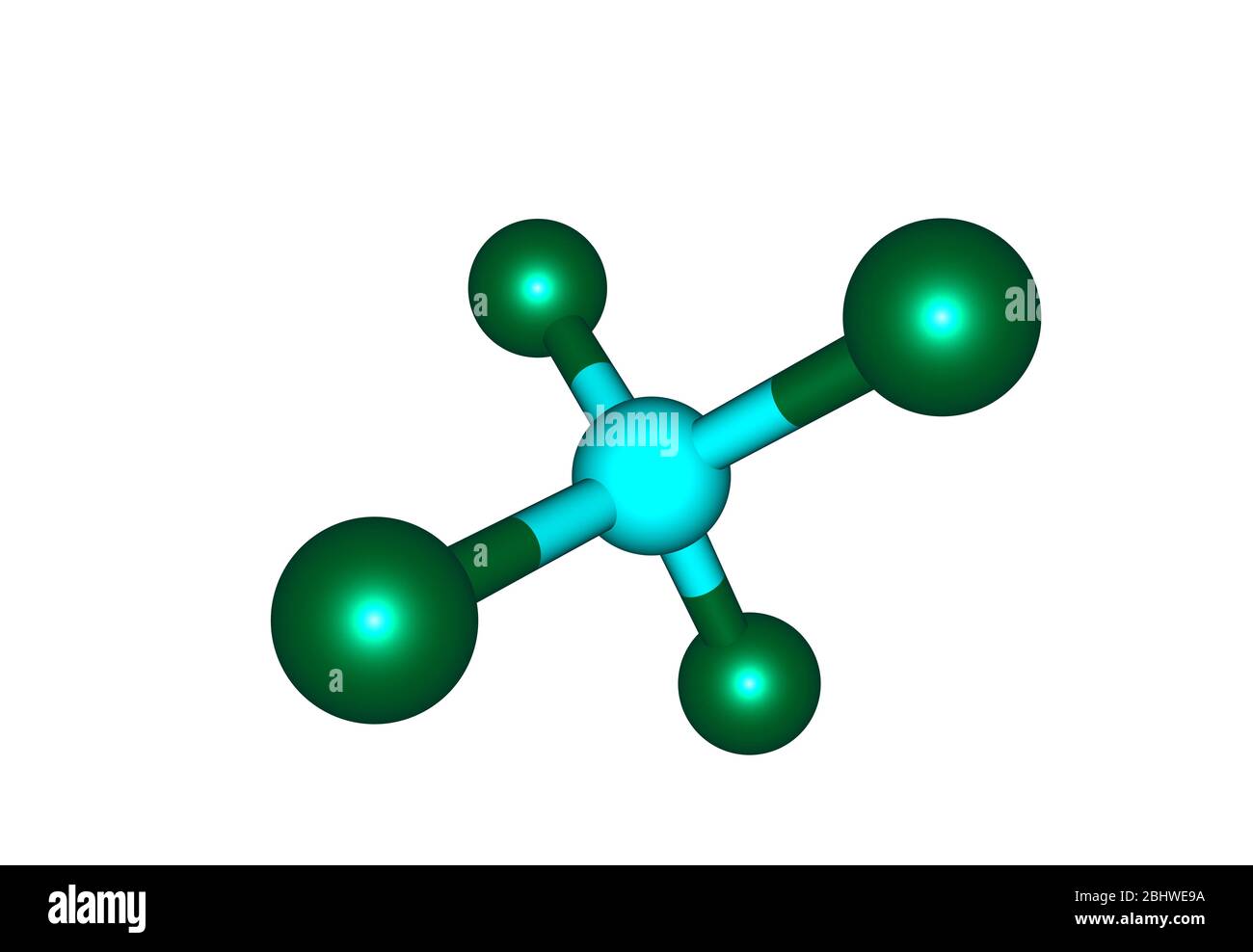
If you’re looking at $CCl_4$ under a microscope, or rather, modeling it in your head, picture a tetrahedron. The carbon sits right in the middle, and the four chlorine atoms hang out at the corners. Because chlorine is way more electronegative than carbon, you’d expect it to be polar. But it’s not. The symmetry is so perfect that the dipoles cancel each other out. It’s a nonpolar liquid that doesn't like water but absolutely loves dissolving fats and oils. That one specific trait made it a superstar in the early 20th century.
Understanding the $CCl_4$ Chemical Structure
The formula for carbon tetrachloride tells us exactly how it's built, but it doesn't tell us how it behaves. At room temperature, it’s a clear, heavy liquid. It smells sweet—kinda like chloroform, its chemical cousin. In fact, if you swap one of those chlorine atoms for a hydrogen atom, you literally get chloroform ($CHCl_3$).
Why does the structure matter? Because $CCl_4$ has no C-H bonds. That makes it non-flammable. For a long time, that was its biggest selling point. Back in the day, people used it in "fire grenades." These were glass globes filled with the liquid that you’d hurl at the base of a fire. The glass would shatter, the liquid would vaporize, and it would basically smother the flames by displacing oxygen and inhibiting the chemical chain reaction of the fire.
It was a literal lifesaver. Until it wasn't.
The problem is that when $CCl_4$ hits high heat, it can turn into phosgene gas. If you know your history, you know phosgene was used as a chemical weapon in World War I. So, while you were putting out your kitchen fire, you might accidentally be gassing yourself. Not great.
From Dry Cleaning to the Ozone Hole
Honestly, the rise and fall of carbon tetrachloride is a wild ride. For decades, it was the king of dry cleaning. If you had a grease stain on your Sunday best in 1940, the formula for carbon tetrachloride was the secret weapon that got it out. It was cheap to produce and worked like a charm.
But the toxicity started catching up with the industry.
Workers in factories and dry cleaners started getting sick. We’re talking serious liver damage (hepatotoxicity). The liver tries to process the $CCl_4$, but it ends up creating highly reactive free radicals. These radicals tear apart the liver cell membranes. It’s so effective at damaging the liver that scientists actually use it in labs today to induce liver injury in test subjects to study how to treat cirrhosis.
Then came the environmental blow.
Carbon tetrachloride is a chlorofluorocarbon (CFC) precursor and a potent greenhouse gas. More importantly, it’s an ozone-depleting substance. When it drifts up into the stratosphere, UV light breaks those C-Cl bonds. This releases chlorine atoms, which then go on a rampage, destroying ozone molecules. This realization led to the Montreal Protocol in 1987. Most of the world agreed to stop using it for consumer goods.
How It’s Actually Made Today
You can't just find a pool of this stuff in nature. It's strictly man-made. Most industrial production involves the chlorination of methane.
$$CH_4 + 4Cl_2 \rightarrow CCl_4 + 4HCl$$
This happens at high temperatures, usually between $400°C$ and $500°C$. It’s a series of steps where hydrogen atoms are replaced by chlorine one by one. You get a mix of methyl chloride, dichloromethane, chloroform, and finally, carbon tetrachloride. They use distillation to separate the "soup" into pure components.
Another way involves the chlorination of carbon disulfide ($CS_2$). It’s an older method but still relevant in certain industrial niches. Even though you can't buy it at a hardware store anymore, it's still produced in massive quantities as a "feedstock." Companies use it to make refrigerants and other chemicals in closed-loop systems where it (hopefully) doesn't leak into the atmosphere.
Real World Safety and Exposure
Is it dangerous? Yeah. Very.
If you somehow come across an old fire extinguisher or a vintage bottle of "cleaning fluid" in your grandma's basement, don't open it. The formula for carbon tetrachloride is linked to:
✨ Don't miss: Why the Apple Store Downtown Los Angeles CA is Not Your Average Phone Shop
- Central Nervous System Depression: Breathing it in makes you feel drunk, dizzy, and eventually unconscious.
- Chronic Liver Damage: As mentioned, it's a "classic" hepatotoxin.
- Kidney Failure: It can shut down your renal system after high exposure.
- Carcinogenicity: The IARC (International Agency for Research on Cancer) classifies it as Group 2B—possibly carcinogenic to humans.
In 2026, the monitoring of $CCl_4$ is actually getting more intense. Even though it's banned for most uses, atmospheric sensors still pick up "unexplained" emissions. Scientists like those at NOAA (National Oceanic and Atmospheric Administration) track these spikes. Sometimes it's a leak from a factory; other times it's an accidental byproduct of other chemical processes. It stays in the atmosphere for about 44 years. That’s a long time for a "banned" chemical to hang around.
Identifying the Substance: Fast Facts
If you're studying for a chemistry exam or just curious, here's the "spark notes" version of what makes this molecule unique.
The molar mass is roughly $153.82\ g/mol$. That’s heavy for such a small molecule. It’s dense, too—about 1.59 times the density of water. If you pour it into a glass of water, it’ll sink straight to the bottom like a stone. It boils at $76.7°C$, which is relatively low, explaining why it evaporates so fast and why those old fire grenades worked so quickly.
It’s also important to note that it has zero flash point. It literally cannot catch fire. This irony is what made it so popular for so long—it was the "safe" chemical that turned out to be incredibly unsafe for the planet and the human body.
Moving Forward: What You Should Do
Understanding the formula for carbon tetrachloride is more than just a chemistry lesson. It’s a cautionary tale about industrial progress. We found a "miracle" solvent that didn't burn, only to realize decades later that it was poking holes in the sky and scarring our livers.
Actionable Insights for the Curious or Concerned:
- Check Vintage Goods: If you collect antique fire extinguishers (the glass "grenade" style) or old cleaning kits, be aware they likely contain $CCl_4$. If one breaks, evacuate the room immediately and call a hazmat professional. Do not vacuum it up.
- Verify Solvent Labels: In industrial settings, always check SDS (Safety Data Sheets) for the CAS number 56-23-5. That's the unique ID for carbon tetrachloride.
- Support Monitoring: Stay informed about the Montreal Protocol updates. The "unexplained emissions" of $CCl_4$ are a major topic in atmospheric science right now, and public awareness helps push for tighter industrial regulations.
- Education: Use the tetrahedral model of $CCl_4$ to understand nonpolar molecules. It’s the perfect example of how high electronegativity doesn't always lead to a polar molecule.
The story of $CCl_4$ is basically finished in terms of consumer use, but its ghost still haunts our atmosphere. Respect the chemistry, but definitely keep your distance.