Ever looked at your phone and wondered why it actually works? It’s not just software or "magic" hardware. It’s physics. Specifically, it’s about a number so incredibly small that our brains literally cannot visualize it. We’re talking about the mass of electron.
It’s tiny.
How tiny? If you took a paperclip and tried to balance it against electrons, you’d need octillions of them. Honestly, the scale is ridiculous. But if that number were even slightly different, the universe would basically fall apart. Atoms wouldn't stay together. Your morning coffee wouldn't exist. You wouldn't exist.
What is the mass of electron exactly?
Scientists have spent decades obsessing over this. Because we can’t exactly put a single electron on a kitchen scale, we use complex experiments involving Penning traps and fundamental constants.
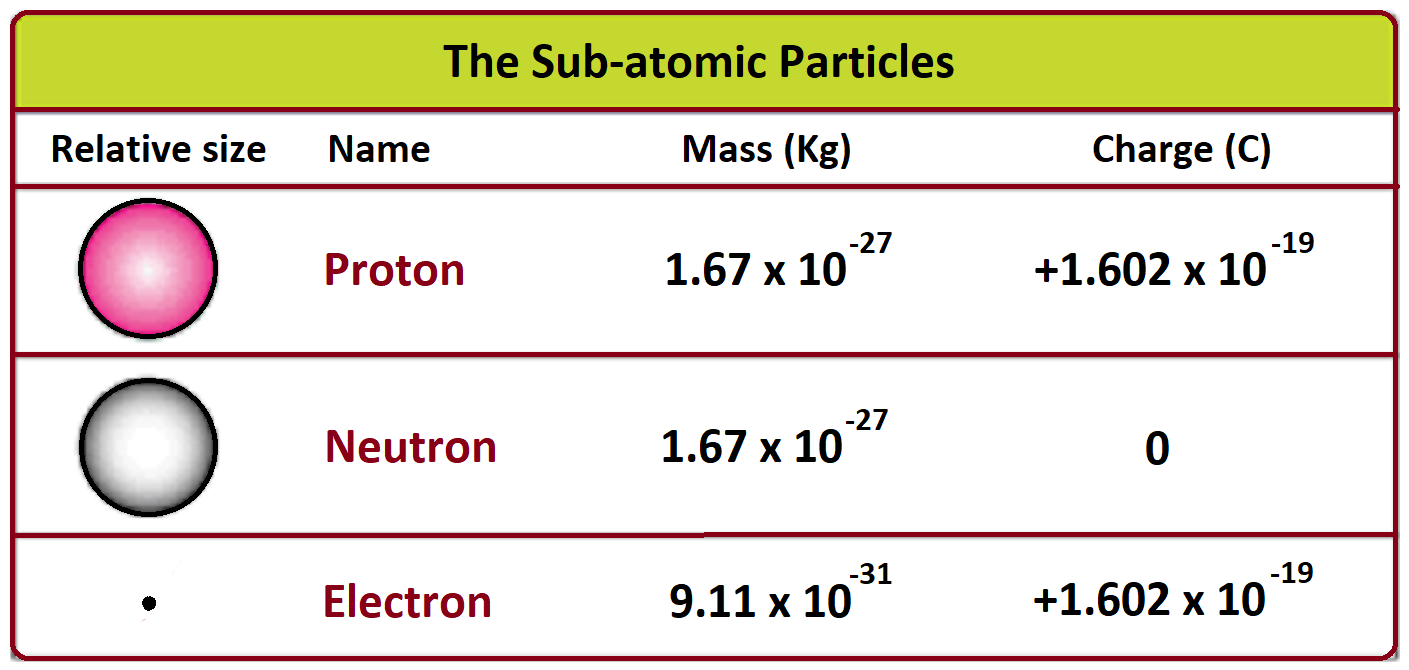
The accepted value for the mass of electron is approximately $9.1093837 \times 10^{-31}$ kilograms.
💡 You might also like: How Can I See My Apple Pay Transactions? The Easier Ways You Might Be Missing
If you prefer atomic mass units (u), which scientists use to keep the numbers from having too many zeros, it's roughly $0.00054858$ u.
Think about that scientific notation for a second. That $-31$ means you move the decimal point thirty-one places to the left. It’s a decimal point followed by thirty zeros and then the nine. It is the definition of "negligible" in everyday conversation, yet it is the heavy lifter of the subatomic world. While protons and neutrons sit in the nucleus acting like the heavy anchors, electrons are the zippy, lightweight messengers that handle all the chemistry.
Why the mass of electron isn't just a boring stat
You might think, "Okay, it’s small, who cares?"
Well, the mass of electron is a fundamental constant of nature. In physics, we often talk about the "fine-structure constant," which dictates the strength of electromagnetic interactions. The electron's mass is a key ingredient in that recipe. If the electron were as heavy as a proton, atoms would collapse instantly. The lightweight nature of the electron allows it to exist in a "cloud" around the nucleus, creating the volume of the atom.
Most of an atom is empty space. The electron defines the boundaries of that space.
The Comparison Game
To put it in perspective, a proton is about 1,836 times heavier than an electron. Imagine a basketball sitting next to a small grape? No, that's not even close. It’s more like a bowling ball sitting next to a single penny. Or better yet, a massive 100lb dog compared to a tiny 1-ounce sparrow.
This massive weight discrepancy is why electricity works. Because electrons are so light, they are incredibly easy to move. When you flip a light switch, you aren't moving protons; you're shoving these lightweight electrons through a copper wire. Their low mass means they have low inertia, allowing them to respond almost instantaneously to electric fields.
How do we even weigh something that small?
You can't see it. You can't touch it. So how do we know the mass of electron with such terrifying precision?
It started with J.J. Thomson back in 1897. He didn't find the mass directly; he found the charge-to-mass ratio ($e/m$). He used cathode ray tubes—those big, bulky glass things that used to be inside old TVs. By watching how the "rays" bent in magnetic and electric fields, he realized these weren't just waves; they were particles with very little mass.
Later, Robert Millikan did his famous oil-drop experiment. He sprayed tiny drops of oil, charged them with X-rays, and hovered them between two metal plates. By balancing gravity against electricity, he found the charge of a single electron.
Once you have the charge ($e$) and the ratio ($e/m$), the math is basically middle-school level. You just solve for $m$.
$$m = \frac{e}{(e/m)}$$
Today, we use the CODATA (Committee on Data for Science and Technology) recommended values. These are updated every few years as our lasers and magnetic traps get more precise. The current precision is staggering—we know this mass to more than eight decimal places.
The Quantum Headache: Rest Mass vs. Relativistic Mass
Here is where things get weird. When we talk about the mass of electron, we are usually talking about its "rest mass." This is the mass it has when it's sitting still (which, to be fair, electrons almost never do).
According to Einstein’s Special Relativity, as things move faster, they sort of "gain" mass. Not literally gaining more "stuff," but their resistance to acceleration increases. In high-energy particle accelerators like the Large Hadron Collider (LHC), electrons can be pushed to speeds incredibly close to the speed of light. At those speeds, their effective mass balloons.
But for almost every practical application—chemistry, electronics, biology—we stick to the rest mass. It's the "invariant" mass that defines the particle's identity.
Common Misconceptions about Electron Weight
People often get confused about a few things:
- "It's so small it's zero." Nope. If it were zero, like a photon, it would have to travel at the speed of light and couldn't be trapped in an atom. Mass is what allows electrons to stay "local."
- "Mass and size are the same." Actually, in quantum mechanics, the electron is considered a "point particle." It technically has no physical volume or radius. It has mass, but it doesn't "take up space" in the way a marble does. It’s a point of mass and charge.
- "Electrons weigh the same everywhere." Mostly yes, but their potential energy in an atom can change how they behave. However, the fundamental mass of electron is a constant throughout the observable universe.
The Technological Impact
Everything you do online depends on the mass of electron.
📖 Related: Semiconductor Chip Tariff Trump: Why Your Laptop Is About to Get Way More Expensive
Semiconductors work because we can precisely control how these tiny masses move through silicon. In a transistor, we use a small voltage to "gate" the flow of electrons. Because their mass is so low, we can switch them on and off billions of times per second (that’s your GHz CPU speed).
If electrons were heavier, your computer would run at the speed of a snail and probably melt from the friction/energy required to move the heavier particles. We owe our entire digital age to the fact that the electron is a cosmic featherweight.
Understanding the math in your head
If you want to grasp the scale, try this mental exercise:
If an electron weighed as much as a 1-gram paperclip, a person (70kg) would weigh as much as the entire Earth. Actually, even more. The scale of the subatomic world is almost impossible to grasp without these weird analogies.
Actionable Insights for Students and Tech Enthusiasts
If you are studying for a physics exam or just trying to understand the hardware under your hood, keep these points in mind:
- Memorize the Power of Ten: It's $10^{-31}$ kg. Remember "31" as the number. It's the most important part of the value for quick calculations.
- The 1/1836 Rule: Always remember that the electron is roughly 1/1800th the mass of a proton. This explains why the nucleus is the center of gravity for an atom while electrons do the moving.
- Check the CODATA: If you are doing high-level research, don't rely on textbooks from the 90s. The value for the mass of electron is refined every few years. Always pull the latest "CODATA recommended value" for the highest precision.
- Context Matters: In solid-state physics, scientists often talk about "effective mass." This isn't the actual mass of the electron; it's a way to simplify how an electron moves through a crystal lattice. Don't confuse the two!
The mass of electron isn't just a number in a textbook. It's the specific setting on the universe's control panel that allows chemistry to happen, light to be emitted, and your brain to send electrical signals to your hands. It's the smallest thing that makes the biggest difference.