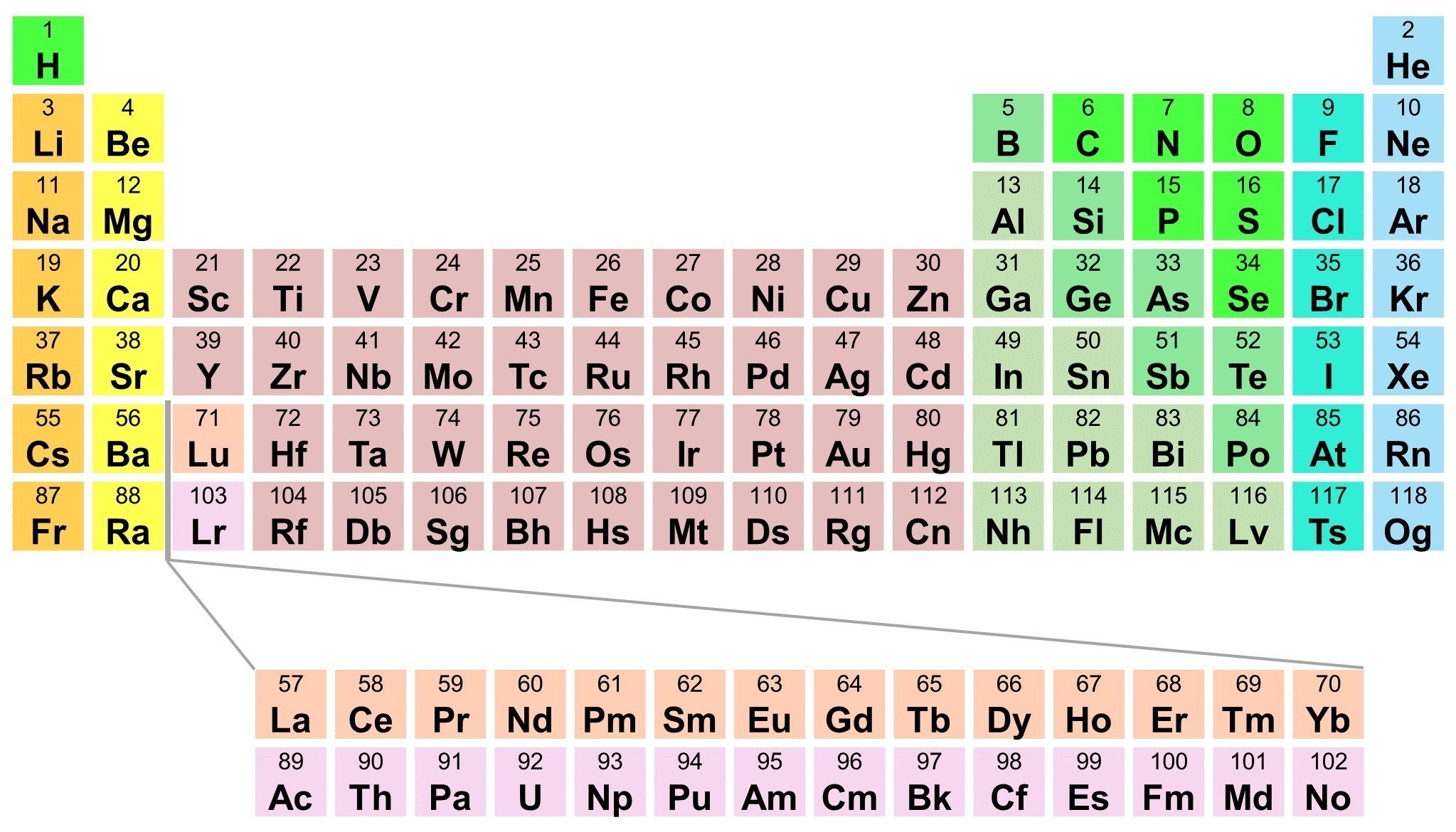
You’re staring at a periodic table. It’s a mess of boxes, letters, and numbers that look like a math textbook exploded on the wall of your chemistry classroom. You need to find one specific thing, but the labels aren’t exactly shouting at you. So, where is the atomic number?
If you’re looking at a standard cell for an element—let's say Carbon—you’ll usually see a whole number sitting right at the very top. It’s almost always above the element symbol. In Carbon’s case, that number is 6. That isn’t just a random filing digit; it’s the most important piece of information about that atom. It tells you exactly how many protons are stuffed into the nucleus. If you change that number, you change the element. Turn that 6 into a 7, and you don't have Carbon anymore; you have Nitrogen. It’s basically the element's social security number.
The Anatomy of the Periodic Table Square
Most people get tripped up because different publishers like to move things around to be "creative." But 99% of the time, the layout follows a predictable hierarchy. The atomic number takes the penthouse suite. Below that, you’ll see the chemical symbol (like C, H, or Au). Underneath the symbol, you usually find the element name, and at the very bottom, there’s a decimal number. That decimal is the atomic mass, and you definitely don't want to confuse the two.
Why does the placement matter? Because the entire periodic table is organized by this value. It’s not alphabetical. It’s not by "vibe." It’s a strictly ascending list. Hydrogen is 1 because it has one proton. Helium is 2. Lithium is 3. It flows like a book, from left to right, top to bottom. If you’re lost, just follow the numbers like a trail of breadcrumbs.
Why Do We Even Call It "Z"?
In serious scientific papers, you might see the letter Z used to represent the atomic number. It seems random, right? Why not "A"? Well, "A" was already taken for the mass number (the total count of protons and neutrons). The "Z" actually comes from the German word Zahl, which literally just means "number." Specifically, it refers to Atomzahl.
Henry Moseley is the guy we have to thank for this. Back in 1913, he used X-ray spectroscopy to prove that the properties of elements aren't just based on how heavy they are, but on the physical charge of the nucleus. Before Moseley, the periodic table was a bit of a guessing game based on atomic weights. Weights can be messy. Isotopes exist. Some versions of an atom are heavier than others. But the proton count? That's immutable. Moseley’s work was so revolutionary that it allowed scientists to predict elements that hadn't even been discovered yet because there were "holes" in the numbering sequence.
Hunting for the Number in Nuclear Notation
Sometimes you aren't looking at a colorful wall poster. You might be looking at a scientific shorthand called nuclear notation or isotope notation. This is where things get slightly annoying because the rules change.
💡 You might also like: Apple Terabyte Hard Drive: Why Everyone Is Still Obsessed With Physical Storage
In this format, you have the element symbol in the middle. To the left, there are two numbers stacked on top of each other. In this specific view, the atomic number is actually the one on the bottom. The top number is the mass number.
- Top Left: Mass Number (Protons + Neutrons)
- Bottom Left: Atomic Number (Just Protons)
It feels backwards compared to the periodic table, but there's a mathematical reason for it. If you want to find out how many neutrons are in an atom, you just subtract the bottom number from the top one. It’s a vertical subtraction problem built right into the notation. Easy.
The Proton Identity Crisis
What happens if you find a number that isn't a whole number? You've found the wrong thing. If you see something like 12.011, that’s the atomic weight. It’s a weighted average of all the different isotopes found in nature. Protons don't come in fractions. You can't have half a proton. So, if the number has a decimal point, keep looking. The atomic number is always, without exception, an integer.
✨ Don't miss: What Does Element Mean: Why We Keep Getting the Basics Wrong
Protons are the anchors. Electrons can come and go—that’s how ions are formed. Neutrons can vary—that’s how you get isotopes. But if the number of protons changes, the identity of the atom dies. It becomes something else entirely. This is why the atomic number is the primary key for the entire universe's inventory system.
Common Places Where People Get Confused
Honestly, the biggest headache comes from "simplified" tables found in some textbooks or online graphics. Some designers think it looks better to put the atomic number in the top right corner. Others put it in the center.
If you're ever in doubt, look for the smallest whole number in the box. Unless you're looking at Hydrogen (where the atomic number and mass are both roughly 1), the atomic number will always be the smaller of the two main numbers listed. For example, in Gold (Au), you’ll see 79 and 196.97. Even if the 79 was written in the basement of the box, you’d know it’s the atomic number because there’s no way Gold only has 79 total particles in its nucleus.
💡 You might also like: 8 square root 8: What Most People Get Wrong About Simplifying Radicals
Practical Steps for Identifying Elements
If you are trying to use this information for a lab or a test, here is the mental checklist you should run:
- Locate the Box: Find your element on the periodic table.
- Find the Integer: Look for the whole number, usually at the top. This is your atomic number.
- Check the Order: Verify that the number is one higher than the element to its left and one lower than the element to its right.
- Identify the Protons: Remember that this number is exactly how many protons are in the nucleus.
- Calculate Electrons: If the atom is neutral (no charge), the atomic number also tells you how many electrons are buzzing around the outside.
The atomic number isn't just a label; it's the fundamental logic of chemistry. Once you know where it is, the rest of the table starts to make a lot more sense. It stops being a wall of random data and starts being a map.
To get better at this, try pulling up a blank periodic table and seeing if you can predict where an element like Silver (Ag) should be based on its atomic number (47). Look for the transition metal block in the middle. Seeing the physical flow of these numbers helps lock the concept into your brain far better than just memorizing a definition.
Actionable Next Steps
- Check your source: Open your chemistry textbook or a reliable online database like the Royal Society of Chemistry. Look at their specific layout to see where they tuck the number.
- Practice subtraction: Find the square for Oxygen. Note the atomic number (8) and the mass (16). Subtract 8 from 16. You now know Oxygen has 8 neutrons.
- Visualize the Nucleus: Draw a small circle and put "6P" inside for Carbon. Draw 6 electrons outside. This visual link between the number on the paper and the physical structure of the atom is the "aha!" moment for most students.
- Use a Dynamic Table: Go to Ptable.com. Hover over different elements and watch how the atomic number stays constant while the "weight" changes as you toggle through isotopes.