You’re holding one. Right now. Whether it’s a smartphone, a laptop, or maybe you’re reading this on a tablet while your EV charges in the garage, you are currently tethered to a lithium ion battery. It’s the invisible heartbeat of the modern world. But honestly, most people have no clue what’s actually happening inside that sleek glass-and-metal sandwich. We just get annoyed when the little percentage icon turns red.
So, what is li ion?
At its simplest, it’s a family of rechargeable battery types where lithium ions move from the negative electrode to the positive electrode during discharge and back when charging. It sounds like high school chemistry because, well, it is. But the impact is massive. Before these things went mainstream in the 90s (thanks, Sony), we were stuck with heavy lead-acid blocks or those "memory effect" nickel-cadmium batteries that died if you didn't treat them perfectly. Lithium ion changed the game because it packs a ton of energy into a tiny space.
The Guts of the Thing: How It Actually Works
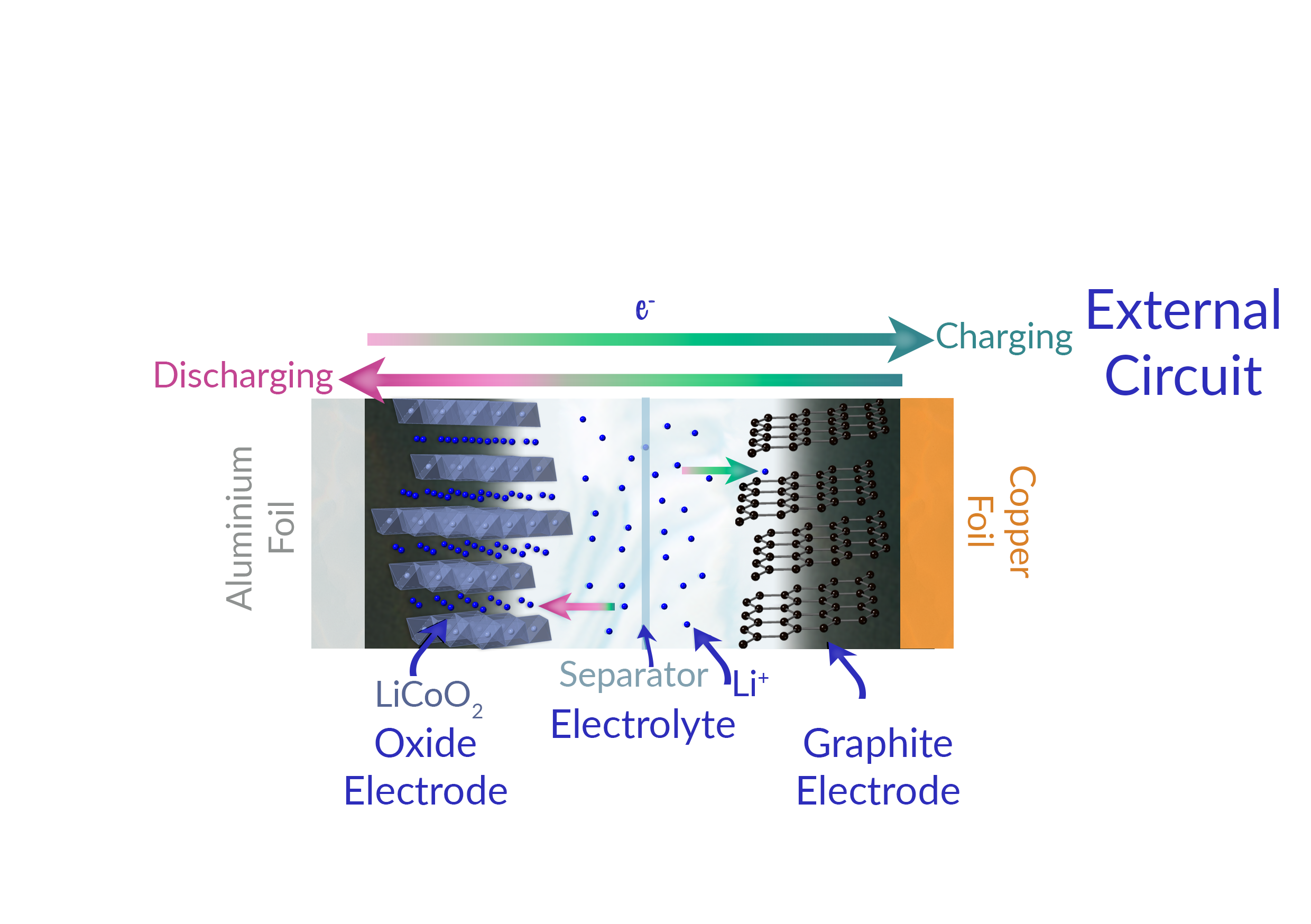
Think of a lithium ion battery like a high-stakes game of musical chairs. Inside, you’ve got an anode (usually made of graphite), a cathode (made of a lithium metal oxide), and a liquid electrolyte that acts as the "floor" the players move across. When you’re using your phone to scroll through TikTok, lithium ions are rushing from the anode to the cathode. This movement creates an electrical current. When you plug it into the wall, you’re basically forcing those ions to run back to the anode to wait for their next turn.
It's a delicate dance.
The reason your phone gets hot? Resistance. Moving those ions through the electrolyte creates heat. If they move too fast or the battery is damaged, things get spicy. We’ve all seen the videos of "thermal runaway." That’s what happens when the internal separator—the thin plastic sheet keeping the anode and cathode from touching—fails. If they touch, it’s a short circuit. The energy releases all at once. Fire. Smoke. Bad times.
Why Lithium?
Why not gold? Or sodium? Or lead?
Lithium is the lightest metal on the periodic table. It also has a massive electrochemical potential. Basically, it’s really good at giving up its electrons. This gives lithium ion batteries a high energy density. In plain English, that means you can store a lot of "juice" without your phone weighing as much as a brick.
There’s also the lack of "memory effect." Older battery types would "forget" their full capacity if you didn't discharge them completely before recharging. Lithium ion doesn't care. You can top it off at 40%, 60%, or 80% without ruining the battery's "brain." That flexibility is why we can have electric cars that we plug in whenever we get home rather than waiting for them to hit zero on the side of the highway.
The Not-So-Pretty Side: Cobalt and Ethics
We can't talk about lithium ion without talking about the messier side of the supply chain. While lithium is the star of the show, the cathode often needs cobalt to stay stable. A huge chunk of the world’s cobalt comes from the Democratic Republic of Congo.
The human rights reports coming out of those mines are, frankly, horrifying. We’re talking about "artisanal" mining where people, sometimes children, dig with their bare hands in dangerous conditions. It’s a massive ethical hurdle for the tech industry. This is why companies like Tesla and Apple are desperately trying to move toward LFP (Lithium Iron Phosphate) batteries or find ways to recycle old ones.
Misconceptions That are Killing Your Battery
"Leave it plugged in overnight and it’ll explode."
No. It won't. Your device has a Battery Management System (BMS) that acts like a bouncer at a club. Once the battery is full, the BMS cuts off the power. The real danger isn't overcharging; it's heat.
Lithium ion batteries hate being hot. If you leave your phone on a sunny dashboard in July, you are literally cooking the internal chemistry. This causes the electrolyte to break down and the capacity to shrink. Another tip? Don’t let it hit 0%. These batteries are "happiest" between 20% and 80%. Deep discharges stress the chemical structure. If you want your laptop to last five years instead of two, stop letting it die every single night.
Different Flavors of Lithium Ion
Not all li ion batteries are built the same. You’ve got:
- Lithium Cobalt Oxide (LCO): These are in your phones. High energy, but shorter lifespan.
- Lithium Manganese Oxide (LMO): Safer, used in some medical tools and power tools.
- Lithium Iron Phosphate (LFP): The "tank" of the battery world. They don't catch fire easily and last forever, but they aren't as energy-dense. You’ll see these in newer EVs and home solar storage.
- NMC (Nickel Manganese Cobalt): The middle ground often used in power tools and E-bikes.
The Future: Is Li Ion On Its Way Out?
Probably not anytime soon. People keep talking about "Solid State" batteries as the "Lithium Ion Killer." Solid-state swaps the liquid electrolyte for a solid one, making it safer and faster to charge. But here’s the kicker: it’s incredibly hard to manufacture at scale.
Toyota and QuantumScape have been "just a few years away" for a decade now. Until they figure out how to make solid-state batteries for the same price as a standard li ion pack, the liquid-filled version is staying king. We are seeing incremental improvements, though. Silicon anodes are starting to replace graphite, which could boost capacity by 20% or more.
Actionable Steps to Make Your Tech Last
Stop treating your batteries like they’re indestructible. They are consumable items. They have a finite number of "cycles"—usually between 300 and 1,000 before they start to noticeably degrade.
🔗 Read more: iPad Air technical specifications: What most people get wrong
- Keep it cool. If your phone feels like a hot pocket, take the case off and stop using it for a minute.
- Use the 20-80 rule. If you're near a charger, plug in at 20%. Unplug at 80% if you don't need the full range.
- Use original chargers. Cheap knock-off bricks from the gas station often have terrible voltage regulation. They can "fry" the BMS or send too much ripple current into the cells.
- Storage matters. If you’re going to put a device away for months, don’t store it at 100% or 0%. Aim for 50%. This keeps the chemistry stable while it sits on the shelf.
- Check for swelling. If your laptop trackpad starts popping up or your phone screen looks like it's lifting, stop using it immediately. That’s a swollen battery, and it’s a fire hazard. Take it to a pro.
The world runs on lithium. It’s the reason we can have a supercomputer in our pockets and cars that don't puff out black smoke. Understanding the chemistry helps you realize that while it’s a miracle of engineering, it’s also a fragile chemical balance that requires a little bit of respect to keep working right.