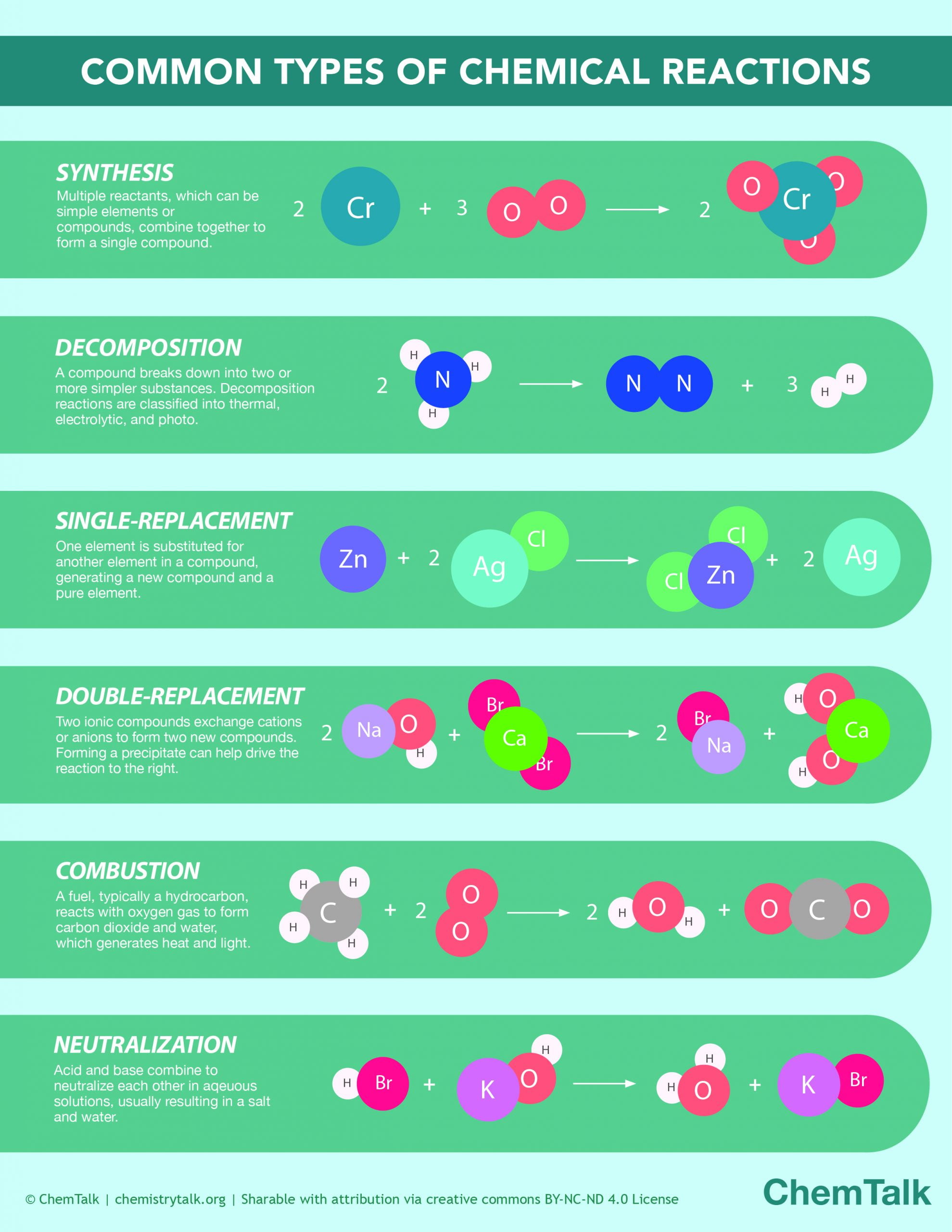
Chemistry is messy. We’re taught in high school that if you mix A and B, you get C. It’s neat. It’s predictable. But honestly, if you’ve ever spent five minutes in a real laboratory or looked at a rusted-out car frame, you know that the product of a chemical reaction is rarely that straightforward. You’re often left with a slurry of side products, unreacted leftovers, and heat you didn't necessarily want.
Think about a campfire. You start with wood and oxygen. You’re looking for heat and light. Those are your desired outcomes. But the actual products? You’re getting carbon dioxide, water vapor, and a pile of ash. If the fire isn't burning quite right because the wood is damp, you suddenly get carbon monoxide—a deadly byproduct. The "product" isn't just one thing. It's an entire suite of molecules rearranged from their original dance partners into something entirely new.
The Reality of Chemical Yields and "Side Effects"
When we talk about the product of a chemical reaction, we have to talk about yield. There’s the theoretical yield—the dream scenario where every single atom does exactly what the textbook says—and then there’s the actual yield. The gap between them is where the real science happens.
In the pharmaceutical industry, this gap is everything. Let's look at something like the synthesis of Oseltamivir (Tamiflu). It’s a complex process. If the chemists get a 90% yield on one step, they’re throwing a party. Why? Because across a twelve-step synthesis, a 90% yield at each stage means you end up with a tiny fraction of what you started with. Most of your "product" ends up as waste or "side products" that have to be meticulously filtered out.
Nature doesn't care about your efficiency.
Sometimes the product of a chemical reaction isn't even a physical substance you can hold. It’s energy. In an exothermic reaction, like the combustion of methane in your stove, the "product" that matters to you is the thermal energy. The $CO_{2}$ and $H_{2}O$ are just the exhaust. Conversely, in endothermic reactions, like photosynthesis, the products (glucose and oxygen) actually store energy that was absorbed from sunlight.
💡 You might also like: My Apple TV Remote Is Not Working: Real Fixes That Actually Work
Catalysts: The Invisible Hand
You can’t talk about products without talking about the things that help make them but don't end up in the final mix. Catalysts.
Basically, a catalyst is like a matchmaker. It brings the reactants together, lowers the energy needed for them to bond, and then leaves the scene completely unchanged. In your car’s catalytic converter, precious metals like platinum and rhodium take nasty products like nitrogen oxides and turn them into harmless nitrogen and oxygen. The metals aren't part of the product of a chemical reaction in a structural sense, but the reaction wouldn't happen at a useful speed without them.
Reversible Reactions: The Products That Don't Want to Stay Products
This is where it gets kinda trippy. Not every reaction goes to completion.
Some reactions reach an equilibrium. It’s a tug-of-war. The reactants turn into products, but the products are simultaneously turning back into reactants. This happens in your blood right now with carbonic acid. It's a constant loop. If you want to maximize the product of a chemical reaction that is reversible, you have to "cheat." You have to remove the product as soon as it forms, forcing the reaction to keep moving forward to fill the vacuum. This is known as Le Chatelier's Principle. It’s the backbone of industrial chemistry.
Why Some Products are Accidents
We’ve all heard of Teflon. It’s the stuff on your non-stick pans. Roy Plunkett wasn't trying to make it. He was working with refrigerants—gases—and one day his canister seemed empty but still felt heavy. He sawed it open. Inside was a white, waxy powder. That "product" was the result of unintended polymerization.
👉 See also: Rufus You Were Right: Why the Amazon AI Shopping Assistant is Actually Changing Things
This happens in nature too. The product of a chemical reaction in the atmosphere can lead to secondary pollutants. You have primary pollutants like $NO_{x}$ from car exhaust, which then react with sunlight and volatile organic compounds to create ground-level ozone (smog). Nobody "manufactured" the smog; it’s a secondary product born from a chaotic environment.
Precision in Modern Synthesis
Today, we are moving toward "Green Chemistry." The goal is "atom economy."
Ideally, every single atom you put into a flask should end up in the final, useful product. If you’re making a plastic and half your ingredients end up as toxic salt water, you’ve failed the atom economy test. Companies like BASF and Dow are obsessed with this because waste is expensive. If the product of a chemical reaction includes a bunch of junk you have to pay to bury in a landfill, your profit margins evaporate.
Think about the Haber-Bosch process. It’s how we make ammonia for fertilizer. It literally keeps billions of people alive. The product is simple: $NH_{3}$. But getting there requires massive pressure and heat. The "cost" of the product isn't just the nitrogen and hydrogen; it's the massive carbon footprint of the energy required to force those atoms together.
Identifying Your Results
How do chemists even know what they’ve made? They don't just look at it and guess.
- Mass Spectrometry: This weighs the molecules. If your product is supposed to weigh 180 grams per mole and your "stuff" weighs 200, you’ve got an extra functional group in there.
- NMR Spectroscopy: This is like an MRI for chemicals. It tells you where the hydrogens and carbons are sitting.
- IR Spectroscopy: This tells you what kind of "bonds" are vibrating in the molecule.
If you’re working at home—maybe you’re brewing beer or pickling vegetables—you’re managing the product of a chemical reaction too. Fermentation is just a series of metabolic reactions where yeast turns sugar into ethanol and $CO_{2}$. If you let it go too long or introduce the wrong bacteria, the product changes. Suddenly you have vinegar (acetic acid).
Actionable Steps for Understanding Your Reactions
Whether you are a student, a hobbyist, or just someone curious about why their silver spoons are turning black (that’s silver sulfide, by the way), understanding products requires a few mental shifts.
- Check the stoichiometry. If you’re following a recipe or a formula, the ratios matter. Too much of one reactant won't give you more product; it just leaves you with a mess of unreacted material.
- Monitor the environment. Temperature and pH are the "knobs" of chemistry. A product that forms at $20°C$ might not form at $40°C$ because the molecules are moving too fast to "stick."
- Look for the "hidden" products. Is there a gas being released? Is the container getting hot? These are clues to what is actually happening at the molecular level.
- Safety first. Some products are unstable. Mixing bleach and ammonia creates chloramine gas. The product of a chemical reaction can be useful, but it can also be lethal if you aren't aware of the compatibility of your reactants.
Chemistry isn't just a subject in a book. It’s the transition of the world from one state to another. Every time you breathe, digest, or even hit "send" on an email (thanks to the lithium-ion reactions in your battery), you are witnessing the creation of new products. Respect the process, account for the waste, and always keep an eye out for the "Plunkett moments" where a mistake becomes a breakthrough.